Solids are composed of atoms, molecules, and ions firmly bound together.
The properties of solids depend on the strength of the chemical bonds within them. Most solids have a crystalline structure.
Their particles – molecules, atoms or ions – are arranged in a strict order. Such a regular structure is called a spatial, or crystalline, lattice. Type of crystalline lattice of solids Ionic structure Molecular structure of solids Atomic structure Metal structure of solids Graphite conductivity is a rare example of a non-metal conductor Interesting experiments with carbon dioxide or hard ice
Type of crystalline lattice of solids The type of lattice depends on what particles are in the lattice sites.
There are 4 main types of spatial gratings – ionic, molecular, atomic and metallic. Particles in crystal lattices are not mobile, or constantly oscillate. With increasing temperature, the vibrational energy of particles of a solid increases, and when it exceeds the energy of intermolecular attraction, the crystal lattice is destroyed – melting occurs. Ionic structure Substances with an ionic structure, for example sodium chloride, usually have rather high melting points.
This property follows from the strong interaction between oppositely charged lattice ions.
molecular, atomic and metallic. Particles in crystal lattices are not mobile, or constantly oscillate. With increasing temperature, the vibrational energy of particles of a solid increases, and when it exceeds the energy of intermolecular attraction, the crystal lattice is destroyed – melting occurs. Ionic structure Substances with an ionic structure, for example sodium chloride, usually have rather high melting points. This property follows from the strong interaction between oppositely charged lattice ions.
Ionic substances are quite fragile
The force exerted by the crystal from the outside can shift the layers of ions, so that equally charged ions will be opposite each other. They will begin to repel, the layers will move apart, and the crystal lattice in this place will collapse.
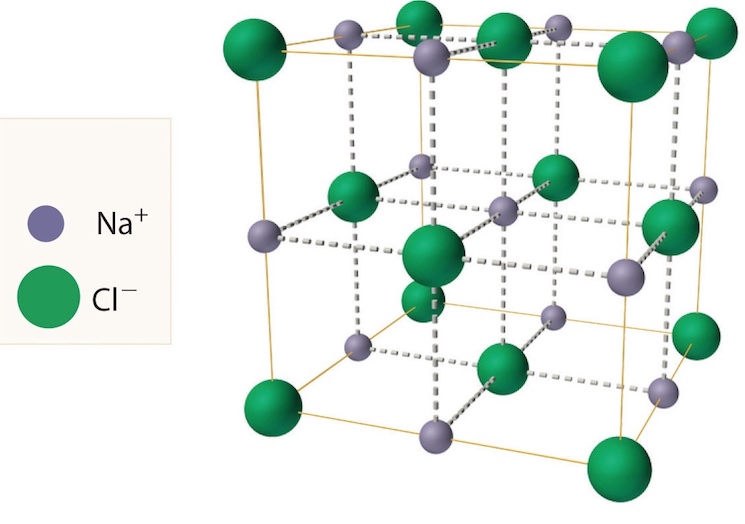
The spatial model of the cubic lattice of the sodium chloride crystal is shown in the figure. Shown here are the relative sizes of the two types of ions and their location in space. Molecular structure of solids Atoms in the atomic lattice. Molecules are composed of atoms bonded by a strong covalent bond. For example, an iodine molecule consists of two atoms linked by the same covalent bond. The bonds between molecules and solids are not so strong. Iodine molecule I2. Solid iodine consists of iodine molecules bound in a regular crystal lattice.
Each iodine molecule consists of 2 iodine atoms firmly bound together.
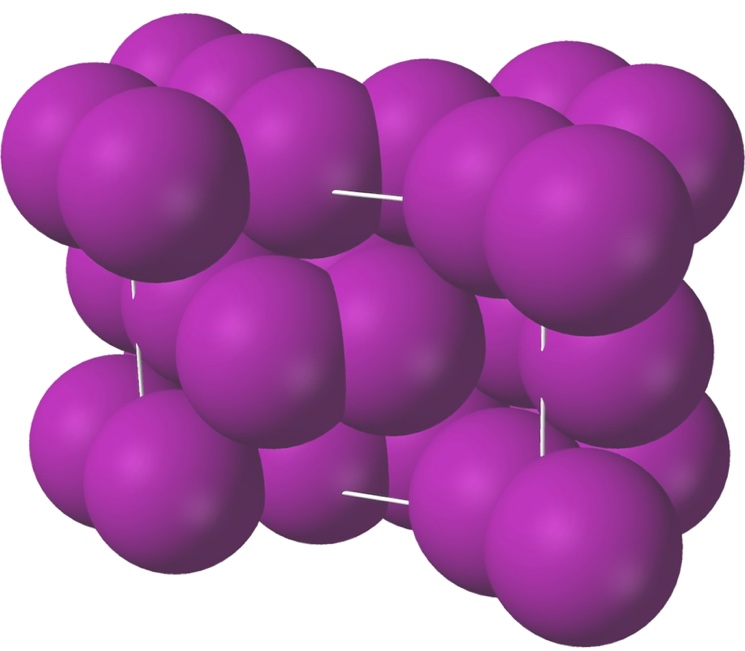
In the solid state, iodine is a rather soft element, since the bonds between its molecules are weak.

Solids with a molecular structure melt, as a rule, at low temperatures.
During melting, covalent bonds do not break, only bonds between weakly interacting molecules are broken. Atomic structure Free carbon is known in two versions – diamond and graphite. Both diamond and graphite consist only of carbon atoms, however, these two substances have completely different structures. In graphite, a carbon atom is connected to 3 other atoms by short strong covalent bonds.
The 4th electron remains free, which determines the electrical conductivity of graphite.
Hexagonal rings form flat layers. The bonds between the layers are rather weak, and the layers can slide one relative to the other. That is why graphite is used as a solid lubricant. In a diamond, each carbon atom is bonded by strong covalent bonds to 4 other atoms. Billions of atoms are connected in a three-dimensional crystal lattice of unusual strength, which makes diamond the hardest known substance.
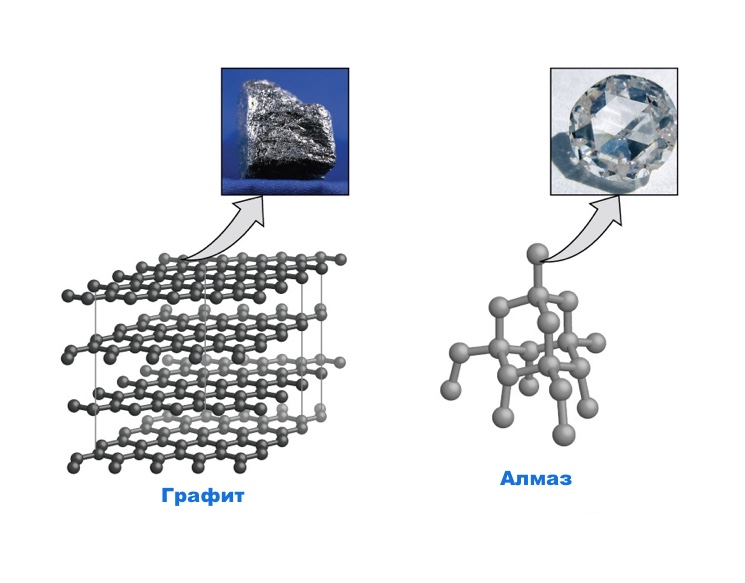
Undoubtedly, diamond is much less common than graphite, and much more valuable than it.
Both diamond and graphite consist only of carbon atoms, however, these 2 substances have completely different structures, and therefore, completely different compounds. The figure shows the structure of the diamond crystal lattice.
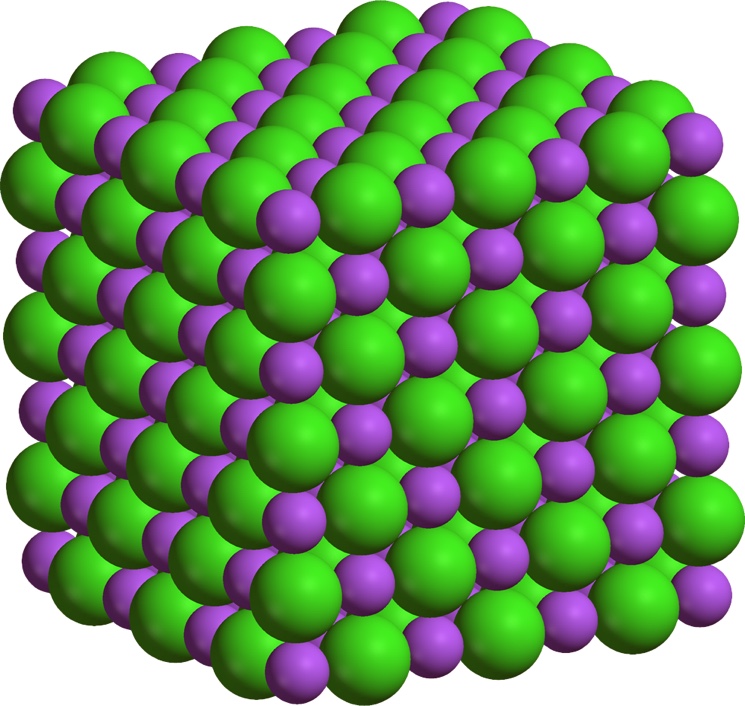
Salt crystals are composed of sodium ions and chloride ions. In the figure, the atoms are shown as balls.
The balls are conventionally spaced so that the three-dimensional structure of the crystal is visible. Pencil lead made of graphite. Weak attractive forces between the layers of carbon atoms allow the layers to slide relative to each other, which is why a graphite trace remains on paper. The metal structure of solids At the sites of substances with a metal lattice are positive ions and metal atoms, and between the nodes are electrons.
Atoms are densely packed in layers, and atoms of one layer are in the deepening of the neighboring layer. The interactions between atoms in such a structure are quite strong, and most metals have high melting points. Many electrons can move freely throughout the metal crystal, and therefore are called free electrons. Free electrons have a negative charge and attract metal cations, as a result of which the crystal lattice of metals is stable.
Free electrons can freely transfer heat and electricity, so they are the cause of the main physical properties that distinguish metals from non-metals – high electrical and thermal conductivity.
Unlike ionic substances, metals are plastic and malleable – metal layers can slip relative to each other without destroying the spatial lattice. Solid metal atoms are tightly packed. External electrons move freely and are evenly distributed between all atoms.
A single electron cloud firmly binds atoms to each other. When an electric current passes through a metal, the total electron flux has a certain direction – from the negative pole to the positive.
Graphite conductivity is a rare example of a non-metal conductor. Electric current is a directed flow of charged particles. These charged particles can be ions or electrons that can move freely. In some cases, the ability of a material to conduct or not conduct electric current allows us to judge its structure.
Graphite is a rare example of a non-metal conductor.
In practice, it is used as conductive “brushes” in a power tool. Graphite conducts current, since each carbon atom in its structure is covalently bonded to only 3 other atoms. Thus, 1 (4) electron at each atom remains relatively free, taking part in the formation of bonds, “Smeared” over the entire layer of atoms. Such a connection is called delocalized.
It is an electron source capable of moving freely through graphite layers to conduct an electric current. An interesting video, which clearly shows not only the conductivity of graphite, but also the formation of an electric arc between graphite rods.
When substances with ionic bonds (salts) are molten or dissolved in water, the crystal lattice is destroyed, ions become free and can conduct electricity.
This phenomenon helped scientists in their time to understand that ionic substances consist of charged particles. Interesting experiments with carbon dioxide or solid ice
An experiment was conducted in the video in which 90 dry ice was poured into an inflatable pool. At -78.5 0С solid carbon dioxide (dry ice) turns into carbon dioxide, bypassing the liquid state. If dry ice is thrown into water, it will begin to evaporate. A mixture of dry ice and water is used for stage effects (thick fog).

